

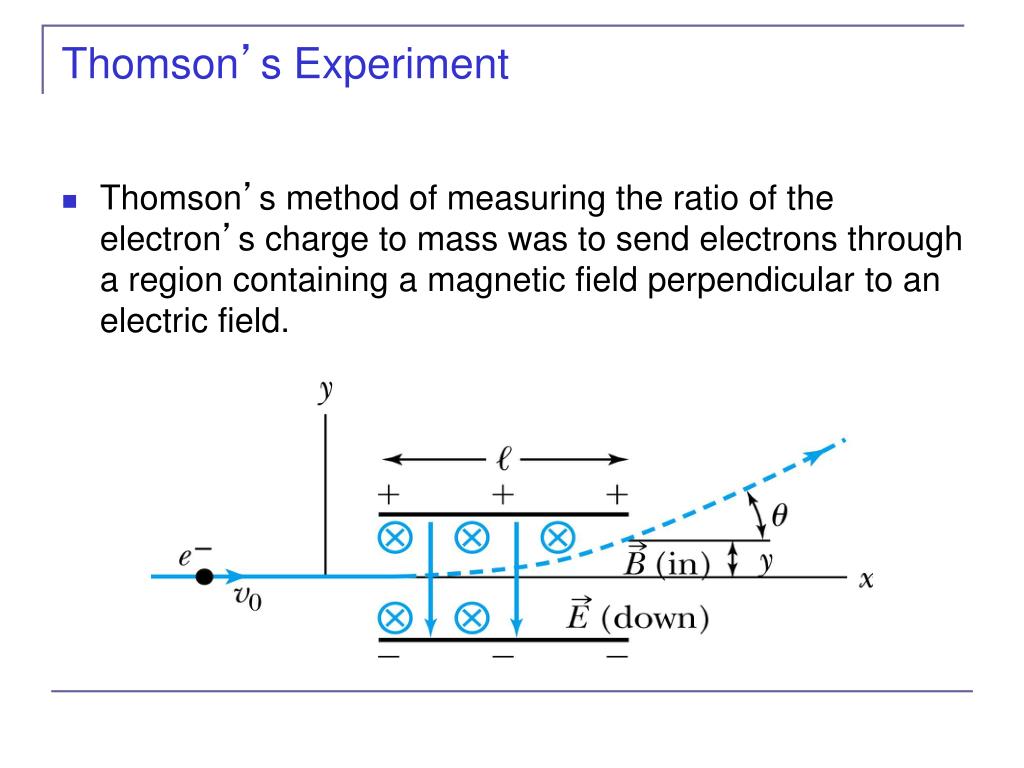
And the significant observation that he made was that the characteristics of cathode rays or electrons did not depend on the material of electrodes or the nature of the gas present in the cathode ray tube. This theory further helped physicists in understanding the structure of an atom. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. So the constituents of the discharge tube were negatively charged.Īfter completing the experiment J.J. By carefully observing the places where fluorescence was observed, it was noted that the deflections were on the positive side.
The electricity starts flowing as the circuit was complete.High voltage is passed to the two metal pieces to ionize the air and make it a conductor of electricity.
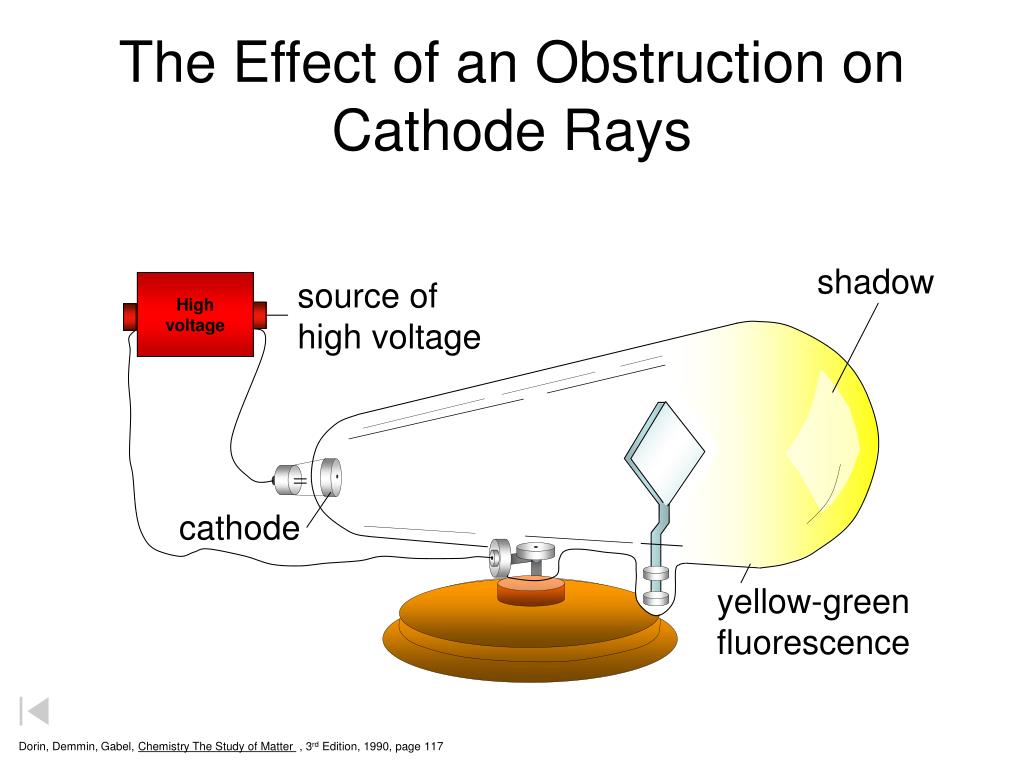
Apparatus is set up by providing a high voltage source and evacuating the air to maintain the low pressure inside the tube.The pressure of the gas inside the tube was lowered by evacuating the air. The two metal pieces were connected with an external voltage. The apparatus of the experiment incorporated a tube made of glass containing two pieces of metals at the opposite ends which acted as an electrode. Light is produced when electrons hit a fluorescent tube.Ī Diagram of JJ.Thomson Cathode Ray Tube Experiment showing Electron Beam – A cathode-ray tube (CRT) is a large, sealed glass tube. The cathode ray tube (CRT), invented in 1897 by the German physicist Karl Ferdinand Braun, is an evacuated glass envelope containing an electron gun a source of electrons and a fluorescent light, usually with internal or external means to accelerate and redirect the electrons. A small amount of energy is transformed into X-rays. When the electrons hit the far end of the tube they give up all the energy they carry due to their speed and this is changed to other forms such as heat. In a cathode ray tube, electrons are accelerated from one end of the tube to the other using an electric field. Cathode rays or streams of electron particles are quite easy to produce, electrons orbit every atom and move from atom to atom as an electric current. The function of the cathode ray tube is to convert an electrical signal into a visual display. A cathode-ray tube (CRT) is a vacuum tube in which an electron beam, deflected by applied electric or magnetic fields, produces a trace on a fluorescent screen.


 0 kommentar(er)
0 kommentar(er)
